Content Map
Heat Transfer to Supercritical Fluids
DOI 10.1615/hedhme.a.000152
2.2.10 Heat transfer to supercritical fluids
I. L. Pioro
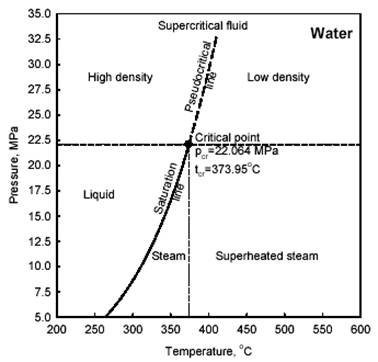
Prior to a general discussion on heat transfer to supercritical fluids it is important to define special terms and expressions used at these conditions. Therefore, general definitions of selected terms and expressions related to critical and supercritical pressures are listed in Part A of this section (for additional details, see also #%SECTION_5.1.16_%#). For better understanding of these terms and expressions a thermodynamic diagram is shown in Figure 1. General definitions of selected terms and expressions related to heat transfer at critical and supercritical pressures are listed in Part D of this section.
A. General definitions of selected terms and expressions related to fluids at critical and supercritical pressures
Compressed fluid is a fluid at a pressure above the critical pressure but at a temperature below the critical temperature.
Critical point (also called a critical state) is the point where the distinction between the liquid and gas (or vapour) phases disappears, i.e., both phases have the same temperature, pressure and volume. The critical point is characterized by the phase state parameters Tcr, pcr and Vcr, which have unique values for each pure substance.
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here