Navigation by alphabet
A B C D E F G H I J K L M N O P Q R S T U V W X Y ZIndex
Assessing lost work in unit operations
DOI 10.1615/hedhme.a.000134
1.9.5 Assessing lost work in unit operations
D. Limb
A. Overview
A unit operation is a sub-element of a complete process, which is defined by a boundary or envelope circumscribing it. Material and energy may cross this boundary. The exergy entering the unit operation envelope via the feed streams or any work done on it will exceed the exergy leaving the envelope by a certain amount. This is the lost work. As we know, unit operations are hooked together in a chain or network to make a complete process. There is a logical direction for the flow of materials and energy — and therefore of exergy through the process. The final elements (unit operations) from which the products are delivered will require a net exergy input to deliver the products under the correct conditions and purities, and to offset the irreversibilities arising within its envelope. The exergy needed by this “final step” must be provided as external work supply or as an input in the streams entering that unit operation’s envelope from preceding unit operations. It thus becomes evident that the irreversibilities in a final step can have a “knock on” effect on the work which has to be done by preceding steps, which themselves are to some extent irreversible — and so on. There is often a compounding effect as one works backwards (in the sense of exergy flow) through the process. This is sometimes expressed by the statement “irreversibilities breed irreversibilities”. The issue is further developed and formalized by Kotas (1986), using the concept Coefficients of Structural Bonds, or CSB. Basically, this coefficient is the ratio of the change in irreversibility rate for the plant as a whole — to the change in a particular component, when one of the design parameters on that component is varied. This concept is intriguing for those wishing to pursue work in the exergy field, but for the rest, it is probably more of academic than of practical interest.
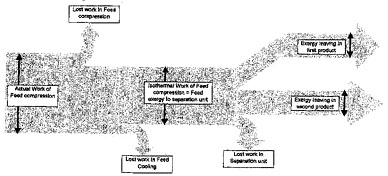
Exergy flow through a process can sometimes be conveniently depicted on a so-called “Grassmann diagram” see Figure 1. This is a development of the “Sankey” diagram used to show heat flow and losses (First Law).
Recently some workers in the field have used exergy as means to assess the overall lifetime costs of a piece of equipment. This takes account not only of the exergy destroyed during its operation but also of the exergy consumed in manufacturing it from raw materials. See Cornelissen (1995).
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here