Content Map
Boiling of Single Component Liquids: Basic Processes
DOI 10.1615/hedhme.a.000191
2.7 BOILING AND EVAPORATION
2.7.1 Boiling of single-component liquids: Basic processes
J. G. Collier and V. Wadekar
A. Vapour formation
The authors thank the United Kingdom Atomic Energy Authority (UKAEA) for permission to use Figure 191.1, Figure 191.3 and Figure 191.4, which remain UKAEA copyright material.
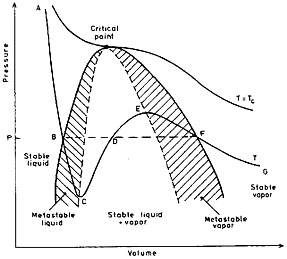
Figure 1 shows diagramatically the pressure-volume isotherms for a pine single-component substance. For a constant temperature T, the pressure and volume vary along a line such as ABFG. Liquid only exists along the line AB, and vapor only exists along the line FG. Liquid and vapor coexist along the line BDF. The saturation curve is the locus of points such as B and F. Corresponding values of the pressure and temperature taken from the curve BDF are known as the saturation pressure (psat) and saturation temperature (Tsat) respectively. So far, only stable equilibrium phase states have been considered. Other metastable or unstable slates can occur. For example, it is possible with care to reduce the pressure imposed on a liquid at constant temperature along a line AB without the formation of vapor at point B. Likewise, it is possible to increase the pressure imposed on a vapor along a line GF without the formation of liquid at F. The coordinates of these metastable states lie along an extrapolation of AB to C or GF to E. Points in the metastable region may also be reached by carefully increasing the liquid temperature above the saturation temperature corresponding to the imposed static pressure; this process is referred to as superheating and the metastable liquid state is referred to as superheated liquid 1.
Vapour and liquid phases can coexist in unstable equilibrium states along lines such : is BC or FE. In this instance the pressures in the liquid and vapor in the vicinity of the interface are no longer equal at equilibrium. If the interface is concave with the center of curvature in the vapor phase, then the vapor pressure (pg) will be greater than the liquid pressure (pℓ) by an amount given by the relationship
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here