Navigation by alphabet
A B C D E F G H I J K L M N O P Q R S T U V W X Y ZIndex
The concept of energy
DOI 10.1615/hedhme.a.000131
1.9.2 The concept of exergy
D. Limb
A. History
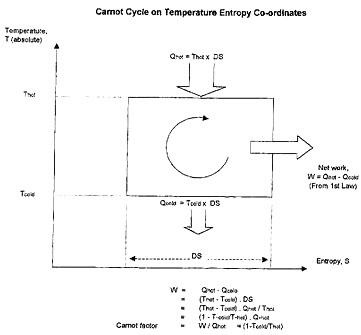
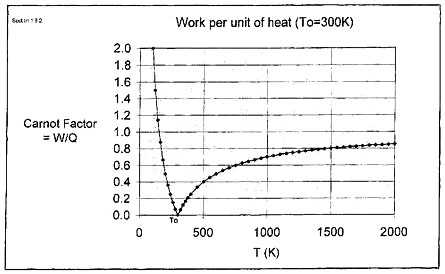
The history of the development of understanding of the nature of heat and work during the 19th century is filled with what are now household names to engineers. These include Ben Thompson (Count Rumford), Clausius, Rankine and many others. Among the most highly acclaimed, Joule had established experimentally in the 1840’s that mechanical energy could be converted without loss into other forms, such as heat, potential energy and kinetic energy, in other words — the First Law. Surprisingly, it was even earlier than this (in 1824) that Carnot among others had realized that the amount of work which can be obtained from a heat engine depended on the temperature levels of the heat source and the heat sink. At that time, it was thought that heat was a substance (caloric) which generated power by passing from a high temperature to a low temperature without itself being destroyed, analogous to power generation in a water turbine where the water itself is not destroyed but where it loses head in creating power. The experiments of Joule, however, showed that, in contrast to the hydraulic turbine case, heat was actually converted to shaft work in a heat engine, albeit at low efficiency. This led to the concept of heat as being energy associated with the vibration of molecules (the “dynamical theory of heat”) as expounded by William Thomson (later Lord Kelvin). Heat can only be partly converted to work, the remainder being discharged from the process as lower grade heat (see Figure 1 and Figure 2). The maximum amount of work which can be obtained from a heat engine in passing unit quantity of heat to or from the environment (at temperature To to a heat source or sink at temperature T ) is known as the “Carnot factor”. As shown in Figure 2, the Carnot factor is zero at T = To. This led to the insight that not all forms of energy had equal “value”. Shaft work was recognized as the highest (most convertible) form of energy.
The term exergy was introduced by Rant in the nineteen-fifties. The concepts upon which it is based — namely the first and second laws of thermodynamics were established by Von Mayer and Carnot in the first half of the nineteenth century. The basis for second law analysis was made by Gouy (1889) and Stodola (1898) by the theorem, which is named after them (Szargut, 1980). A second law analysis was already performed by Stodola in 1905 (Motz, 1989) and by Jouguet (1907). In 1932 Keenan defined the concept of physical exergy, at that time called availability, and used it to analyze a steam turbine. The concept of chemical exergy and its associated reference states has been introduced by Szargut (1986) and Kotas (1995).
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here