Navigation by alphabet
A B C D E F G H I J K L M N O P Q R S T U V W X Y ZIndex
Expressively Clad Plate
DOI 10.1615/hedhme.a.000435
4.5.5 Explosively clad plate
D. G. Nowell, R. Hardwick
A. Principles of explosive bonding
Explosive bonding is a solid-state cold-welding technique originally developed in the United States by Du Pont in the early 1960s. Since that time a number of companies throughout the world have been carrying out explosive cladding of plate and, individually,have further developed and refined the technology. Many of these companies are members of the International Explosive Metalworking Association, which was formed to further the acceptance of the process and provide and maintain uniform standards.
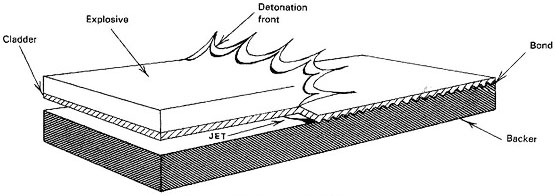
Essentially, the process consists of the use of an explosive charge to create a high impact pressure between the metals being bonded (see Figure 1). The components are initially spaced a small distance apart and are driven together by the explosion so that contact between the two is made progressively over the surface area being joined. At the resulting collision front, the component surfaces are removed as a jet of material containing surface oxides and other contaminants, thereby cleaning the surfaces and permitting the generation of a molecular bond at the interface.
The parameters required to create the correct conditions for bonding have been established for a wide range of material combinations. Some of these metal combinations were found to be more difficult to bond than others, and it appears that this difficulty is related to the atomic spacing and yield strengths of the materials; the greater the differential between the atomic spacings and/or yield strengths of the two metals being bonded, the more difficult bonding becomes. Indeed, where these differentials are small, the metal combination is highly compatible, and it is these metal combinations that can be bonded by the more traditional route where they are merely hot rolled together to produce a bond (roll bonding).
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here