Navigation by alphabet
A B C D E F G H I J K L M N O P Q R S T U V W X Y ZIndex
Properties of Saturated Fluids
DOI 10.1615/hedhme.a.000524
5.5 PHYSICAL PROPERTY DATA TABLES
5.5.1 Properties of saturated fluids
Clive F. Beaton
In this section the thermophysical properties of fluids are presented for the two-phase region — that is to say, from the normal boiling point to the critical point. Data are presented wherever possible from internationally recognised sources. THERMODYNAMIC PROPERTIES are often available from an equation of state representing the PVT behaviour of the fluid, and provide a consistent set of interdependent values. Typical compounds are those listed in Section 525. Data for the properties at the saturation temperature can be derived from theoretical relationships. More usually, however, the ideal gas heat capacity and properties of the saturated liquid below the boiling point are taken from the literature and correlated by methods referred to in Section 5.1 (Tables in Section 533 and Section 534) provide data values for a randomly chosen list of compounds.
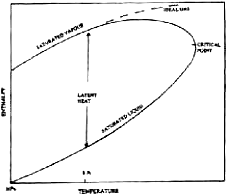
The most generally reliable procedure for obtaining data for the saturated vapour is by the Lee-Kesler generalised equation of state (Lee and Kesler, 1975). The latent heat of vaporisation can be predicted reliably by the Clausius-Clapeyron equation when good vapour pressure and density data are available (Section 500-4). The liquid enthalpy can then be evaluated at pressures above the normal boiling point by difference from the vapour enthalpy. This is represented graphically in Figure 1. This is the method preferred in this revision as it provides a common basis for estimating mixture data. When liquid enthalpies can be derived by integration of the specific heat capacity they are less reliable at temperatures above the normal boiling point.
The TRANSPORT PROPERTIES of many important fluids have been similarly studied, and all such known sources have been consulted. (See Section 537 for specific examples). The properties of liquids can be measured relatively easily, and are well established for many fluids up to temperatures of 0.9Tc. For the saturated vapour, however, few reliable measurements have been made because of inherent experimental difficulties. The generalised procedures of Thodos and co-workers (Jossi et al., 1962; Stiel and Thodos, 1964a; Stiel and Thodos, 1964b) have been used to derive values for the saturated vapour from ideal gas data, using density as the independent variable. Figures on pp. 25 and 27 of Section 526 illustrate the effect of pressure on the properties of steam.
A thorough survey of the liquid viscosity and thermal conductivity of groups of compounds in homologous series has been made by the Engineering Sciences Data Unit over a number of years, and these are used whenever possible. The authors recommend that their equations should not be extrapolated beyond a reduced temperature of 0.9; the tables are therefore limited; in particular liquid thermal conductivity will increase towards the critical point at higher temperatures.
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here