Introduction
DOI 10.1615/hedhme.a.015671
CRYOGENIC HEAT EXCHANGERS
Introduction
G. Venkatarathnam
Cryogenic systems require heat exchangers that operate at very high effectiveness. The need for high effectiveness can be understood using a simple nitrogen liquefier operating on the Linde–Hampson liquefaction process (Figure 1).
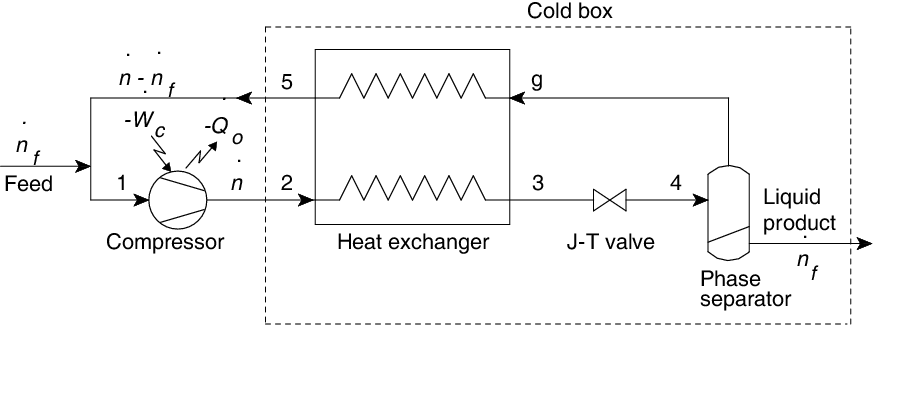
Figure 1 Ideal Linde–Hampson liquefaction process with isothermal compression
The fraction of the gas that gets liquefied on expansion is known as the liquid yield (Y) and can be expressed in terms of the enthalpy of the working fluid in the case of a Linde–Hampson liquefier from an energy balance over the heat exchanger, throttle, and phase separator as follows:
\[\label{eq1} \mbox{liquid yield, }{Y}~=~\frac{\dot{n}_f}{\dot{n}}~=~\frac{h_5-h_2}{h_5-h_f}\;. \tag{1}\]
The liquid yield (Y) can be expressed in terms of the heat exchanger effectiveness as follows:
... You need a subscriptionOpen in a new tab. to view the full text of the article. If you already have the subscription, please login here